| Home
DNA Synthesis
DNA Transcription
Protein Synthesis
Gene Therapy
At a glance
Contact Page
guest book
|
|
|
.........THE PROCESS
TRANSLATION/PROTEIN SYNTHESIS is the process whereby the genetic code (three letter words) is interpreted from information in a nucleic acid sequence into the primary sequence of a polypeptide chain. The process is fairly accurate, making mistakes only once in about ten thousand amino acids incorporated into proteins. Several "proofreading" mechanisms exist to assure this degree of accuracy.Base-pairing is used to match nucleic acid code words (codons in mRNA) with recognition sequences (anticodons) for each amino acid.
Translation requires interactions of over 100 different macromolecules. Thus, the translation system is much more complex than that of replication and transcription, as currently knowledge suggests.
|
|
|
t-RNA & GENETIC CODE
Though the genetic message for protein is brought by the m-RNA....... a protein cannot be synthesised without its constituents namely the amino acids.The amino acids are brought into the region of protein synthesis namely in the ribosomes by t-RNA(TRANSFER RNA).They appear in the shape of aclover- leaf,with protruding arms characterised by the presence of certain nucleotides.The most important of this is the anticodon arm which recognises the triplet sequences in the m-rna and thereby brings in the right amino acids .Here came in the concept of genetic code.It has been identified that the gene is composed of 4 nitrogenous bases namely:A,T,G&C.At the same time there are about 20 amino acids.It was shown thata combination of 3 bases can code for an amino acid.The m-RNAcodon recognition is specified by the tRNA anticodon, not by the actual amino acid on the tRNA .Wobble base-pairing (between the 3' base of codons and the 5'-base of anticodons) allows multiple codon recognition.Codons which differ in the first two bases must be recognized by different tRNAs since these two bases pair normally.
The first (5') base of an anticodon determines how many codons are recognized by a given tRNA [C or A (1 codon), U or G (2 codons), I (3 codons)].
|
|
|
AMINOACYL SYNTHETASES AND RIBOSOMES
Amino-acyl-tRNA synthetase (one for each amino acid) joins amino acids to correct tRNAs (anticodons); also associates amino acids with unstable (high energy) bonds formed by breakdown of ATP; this energy will eventually be used to force formation of peptide bond in growing polypeptide chain. Fidelity of protein synthesis (one mistake in 104 amino acids) relies mostly on the specificity of amino acid and cognate tRNA recognition by these enzymes.
Ribosomes hold together mRNA, tRNA-aa(AMINO ACID); catalyze the formation of peptide bonds to link amino acids together in order.
Ribosomes of E. coli are 70S and consist of a large (50S) and small (30S) subunits.In eukaryotes ribosomes are 80S with 40S and 60S subunits containing (18S) and (5S, 28S and 5.8S) r RNAsRibosomes self-assemble (as does TMV) from mixtures of the rRNA and proteins.
rRNA may have important catalytic roles in protein synthesis--a current hot research area
|
 |
PROTEIN SYNTHESIS
Translation involves 3 major steps
a.Initiation:. The AUG start signal in the m-RNA is preceded by a conserved (Shine-Dalgarno) sequence which base pairs with the 3'-end of 16S rRNA to align the mRNA and the 30S ribosome subunit.
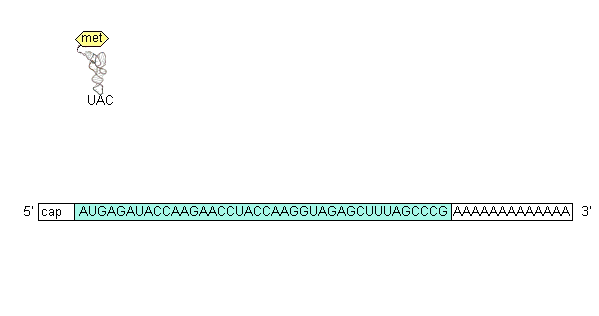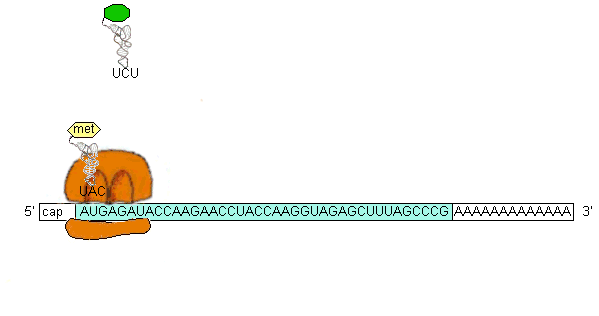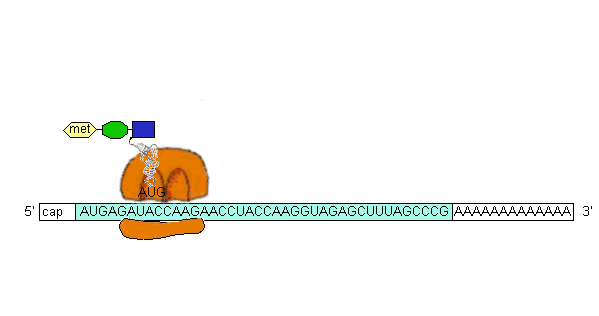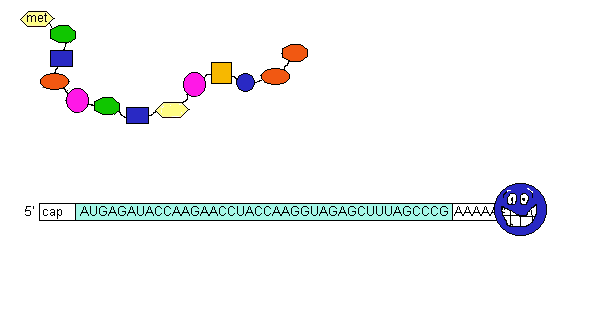
The initiation codon (AUG) base pairs with the anticodon on fMet-tRNAfMet(the RNA containing the amino acid methionine(formyl methionine in prokaryotes)in the P site and, in the presence of GTP and 3 protein initiation factors forms a 30S initiation complex (30S subunit, mRNA, fMet-tRNAfMet.
The 30S initiation complex combines with a 50S subunit [Fig. 34-29] to yield a 70S initiation complex. GTP hydrolysis (to GDP and Pi) and release of IF1 and IF2 also occurs.
2.Elongation
An aa-tRNA (bound to elongation factor EF-Tu) binds to the A site (adjacent to the P site) by base-pairing of the second codon with the aa-tRNA anticodon. The [EF-Tu-aa-tRNA-GTP] complex dissociates with GTP hydrolysis and release of [EF-Tu-GDP] which binds EF-Ts then GTP hydrolysis and EF-Ts dissociation recycles the [Ef-Tu-GDP] to [Ef-Tu-GTP] which then can bind another aa-tRNA for delivery to the ribosome. EF-Tu does not bind to fMet-tRNAfMet, but Met-tRNAMet does bind EF-Tu and is used for all internal Methionine incorporation. Also, IF2 is only used for fMet-tRNAfMet recognition.
The GTPase rate of EF-Tu is rate limiting for protein synthesis and also effectively acts as a proofreading step. Mistakes are reduced by increasing the time (in msec) allowed for checking out the codon-anticodon match. Proofreading occurs before and after hydrolysis of the EF-Tu bound GTP. Error rates are about 1X10-4 giving synthesis of mostly functional proteins.
Peptide bond formation is catalyzed by an intrinsic activity of the 50S ribosome subunit called peptidyl transferase. The fMet from the P site is transferred (by peptide bond formation) to the amino acid of the aa-tRNA in the A site. The uncharged tRNA in the P site is released and the mRNA moves 3 bases in the ribosome thus shifting the dipeptidyl-tRNA to the P site. This process is called translocation. Then a new aa-tRNA occupies the A site and the process repeats. Translocation requires GTP hydrolysis and a protein factor called EF-G.
3.Termination:
Protein synthesis stops when a stop codon is located in the A site and these codons are recognized by release factor (RF) proteins. [RF1 recognizes UAA and UAG; RF2 recognizes UAA or UGA]. After RF binding, peptidyltransferase is activated and the nascent peptide is hydrolyzed from the tRNA, followed by release of the tRNA, mRNA and ribosome subunit dissociation. After binding IF-3, the 30S subunit can participate in another translation cycle.
|
|
|


